Description:
Summary A patented catalyst with the ability to convert waste carbon dioxide and methane into synthesis gas, in a cost-effective and highly efficient manner. Applications include: heat production for industrial purposes, industrial steam, replacement of conventional fuels in boilers, and electricity generation.
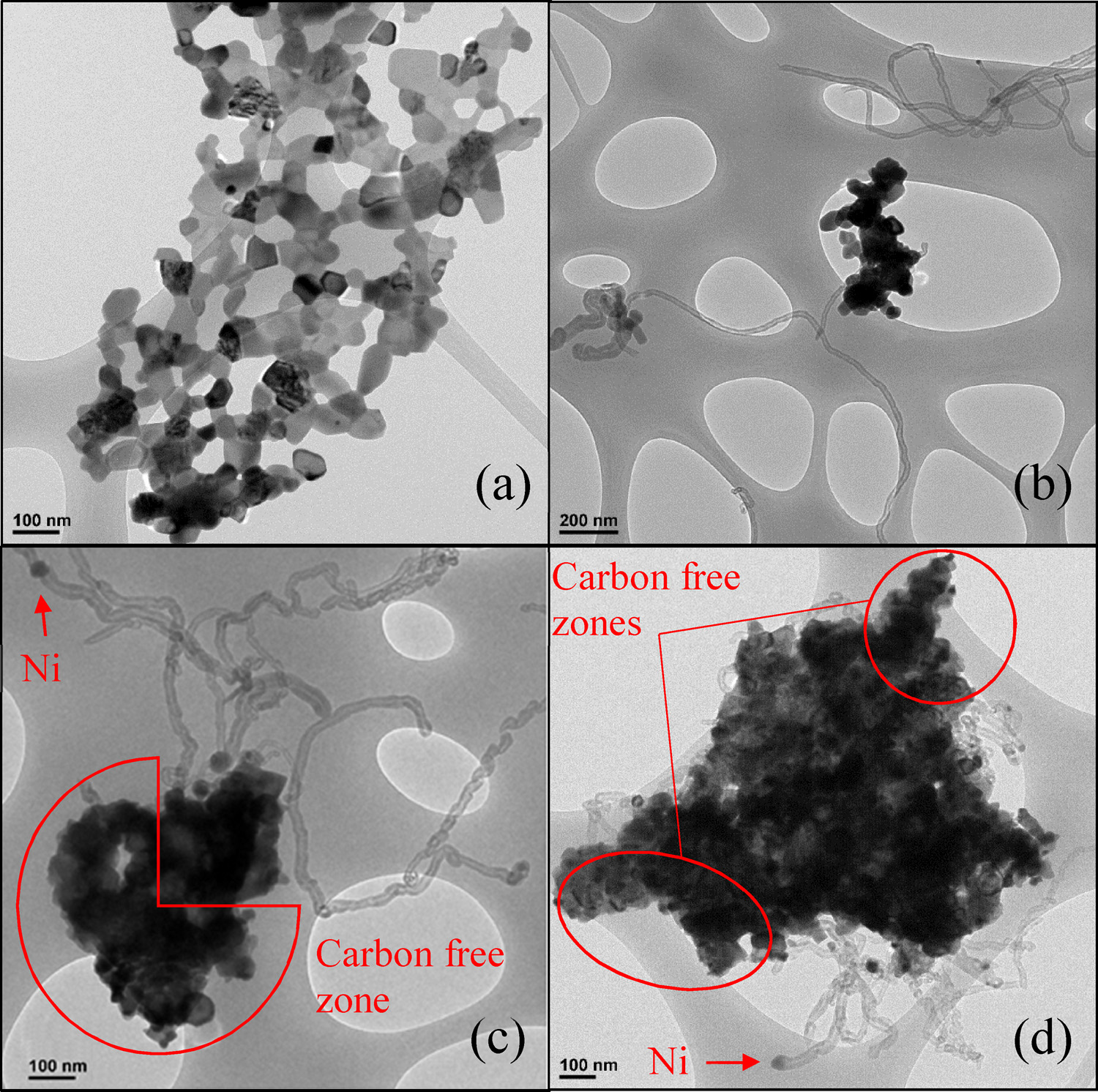
Introduction
The conversion of CO2 into fuels and useful chemicals has been extensively pursued for renewable, sustainable and green energy. Dry reforming is a method of converting CO2 and hydrocarbons (e.g. CH4) into synthesis gas (syngas).
The chemical equation for the reaction is displayed below:
CH4 + CO2⇌ 2CO + 2H2
Owing to the chemical stability of carbon dioxide, the reaction does not occur spontaneously, and needs a catalyst to occur. The catalyst needs to be able to withstand the high temperatures and long operational times required for dry reforming. Currently, most research has centered on the use of catalytic metals supported on a range of high surface area substrates, including aluminas, silicas and mixed metal oxides. However, current catalysts have several limitations, including:
1. Deactivation caused by carbon formation
2. Poor selectivity for hydrogen and carbon monoxide
3. Sintering of active metals, which renders catalysts more susceptible to the deleterious effects of coking.
This has created a prejudice towards noble metal-based catalytic materials, However, high cost of these is seen as a limiting factor for industrial scale-up.
Technology
A joint research group from the University of Surrey and the University of Alicante have developed and patented a solid oxide material suitable for use in catalysing a dry reforming reaction.
The proposed catalyst offers notable advantages compared to previously existing catalysts. It does not only demonstrate a high selectivity for hydrogen and carbon monoxide during hydrocarbon reforming reactions, but it does so at operational temperatures so low that would otherwise lead to deactivation by sintering or coking. Thus, the catalytic materials of the invention allow hydrocarbon reforming to be carried out in a considerably less energy-intensive manner
than with other catalysts. This advantage must be considered alongside the cost saving of not using a noble metal. In summary, the development offers a sizeable development in the industrial-scale reforming of hydrocarbons, such as methane.
Applications/Benefits
• Provides an opportunity to make a profit from waste gases from industrial processes
• Positive implications for climate change and global warming through the offsetting of greenhouse gases
• Can be used as a source of energy for multiple purposes
• Proposed catalyst makes it feasible to scale-up for industrial production
• Provides the selectivity for carbon monoxide and hydrogen as seen with noble metal catalysts, with prices matching those of other non-noble catalytic metals.
• It is highly resistant to deactivation and coking, thus allowing it to participate in the reaction for longer; reducing costs and increasing efficiency.
https://www.surrey.ac.uk/mediacentre/press/2018/surrey-develops-new-%E2%80%98supercatalyst%E2%80%99-recycle-carbon-dioxide-and-methane